CUSTOM ENVIRONMENTAL CONTROL
When it comes to environmental control requirements, no two applications are the same. That's why we're here. We specialize in designing custom solutions to match our client's unique requirements and restrictions.
Temperature Control
Control environments from 0°F to 100°F (-18°C and 38°C) with tolerances as precise as ±0.02°C
Humidity Control
Control areas from trace moisture to 85% RH with tolerances as precise as ±0.5% RH
Filtration
HEPA, ULPA, or molecular
Environmental Control Units
In addition to designing fully custom solutions, Air Innovations has a suite of standardized environmental control products across different industries.
-
Cleanroom Environmental Control Units
Cleanroom Environmental
Control UnitsAir Innovations cleanroom environmental control systems are designed to meet industry standards and stringent process requirements by providing optimal control over the temperature, humidity, air pressure, and airflow rate of a cleanroom. We offer standardized solutions or fully customized systems, depending on the complexity of your application.
Learn More -
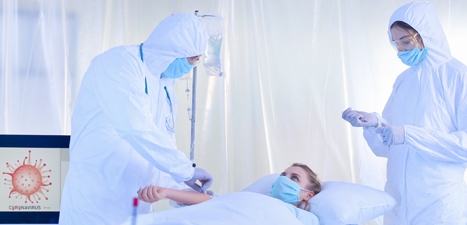
Healthcare Solutions for Post Sterilization and Isolation Spaces
Healthcare Solutions for Post Sterilization and Isolation Spaces
We produce multiple standardized solutions to help keep employees and patients safe in healthcare environments. Our product offerings include units that can maintain sterility, create negative or positive pressure environments, and reduce indoor air pollutants.
Learn More -
MyZone® Desk Console Management System
MyZone® Desk Console Management System
The MyZone desk console management system combines environmental control (heating and cooling), lighting control, and integrated desk-leg lift control into a single unit to help create a comfortable and personalized workspace for employees.
Learn More -
Wine Guardian® Wine Room Cooling Units
Wine Guardian® Wine Room Cooling Units
Wine Guardian, an Air Innovations brand, provides low-temperature HVAC systems for the long-term storage of wine. These units can also be used to control the temperature and humidity conditions for fur storage, art preservation, humidor rooms, and computer system rooms.
Learn More -
Cabin Cool Personal Forklift Cabin AC System
Cabin Cool Personal Forklift Cabin AC System
To keep forklift and industrial equipment operators safe from extremely hot and humid conditions, Air Innovations designed the Cabin Cool system. This personal AC system attaches to a forklift cabin and blows cool air directly onto a vehicle operator.
Learn More
FEATURED CASE STUDIES
Companies across of the world have trusted Air Innovations to deliver solutions for their environmental control challenges. Learn how we do it.
View All Case StudiesRESOURCES
We have produced an extensive resource library to help you gain a deeper understanding of our expertise and capabilities.
Click on a link to below to view resources.

I'm really impressed with the how the unit is responding to the test parameters, and the recovery times are exceptionally impressive. Your attention to detail and expertise is very evident with how the PECS is performing during this final test phase.
ABOUT US
At Air Innovations, we are driven by a history of innovation and guided by a dedicated management team to ensure the reliability and quality of our products.
 Learn More About Us
Learn More About Us
We are a world leader in producing environmental control systems for processes that can't be solved with standard HVAC equipment. Learn more about our expertise in temperature, humidity, and filtration control.
Learn More
 Management Team
Management Team
Our management team is made up of mechanical engineers, MBAs, and even a chemical engineer — all with different backgrounds to help lead our company forward.
Learn More
 Psychometric Testing Facility
Psychometric Testing Facility
Our custom psychrometric testing facility is a dual-chamber laboratory dedicated to testing the safety, reliability, and performance of our products in a controlled environment.
Learn More