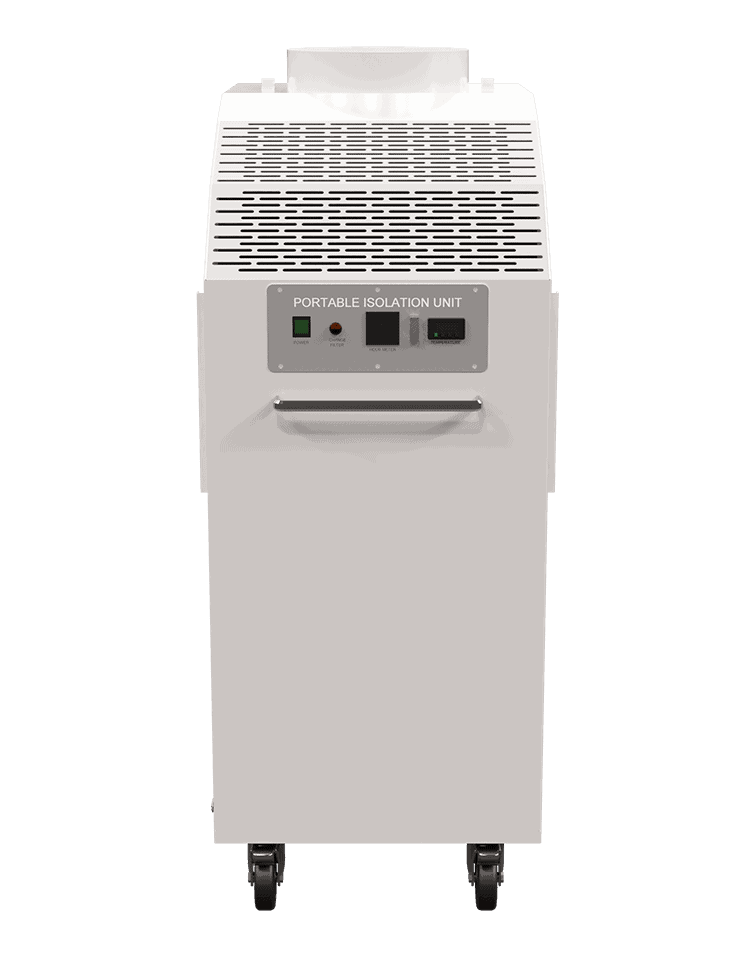
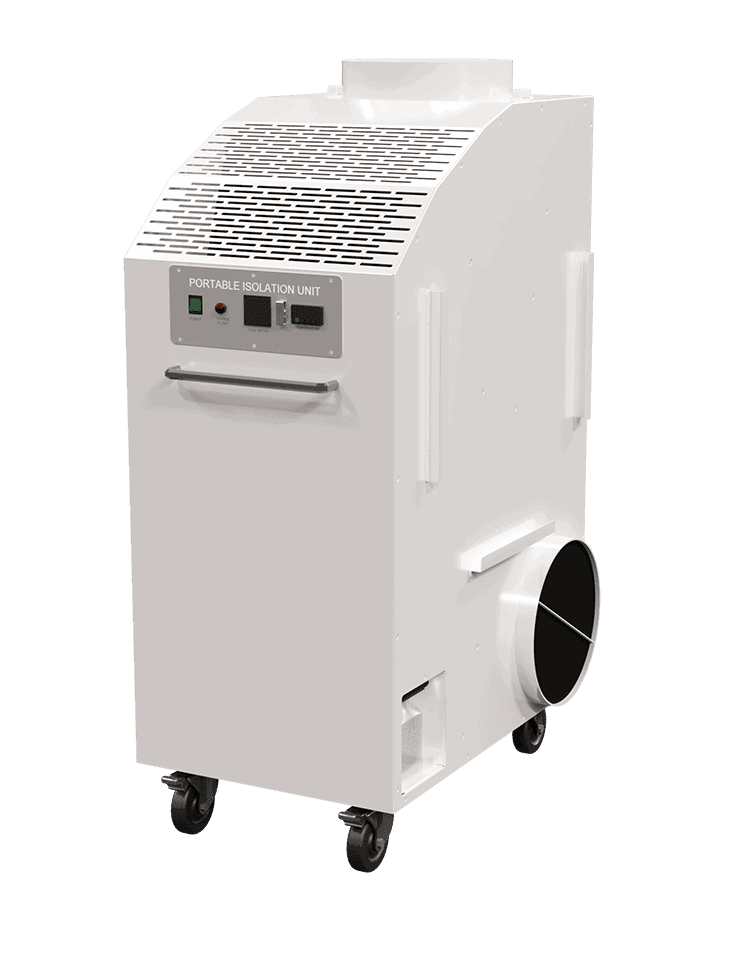
Description
Beyond the Basics – Achieving Contamination Control in Critical Spaces
Combining known technologies into an easy-to-deploy portable unit
- HEPA filtration for airborne particulate removal via recirculated air
- Ultraviolet C (UVC) light to aide in sterilizing airborne viruses and bacteria trapped in the HEPA filter
- Pressure control either negative or positive
- Airborne Infection Isolation Rooms (COVID-19, TB, SARS, smallpox, etc.) require negative pressure
- Protective Environments (burn, immuno-suppressed) require positive pressure
- Temperature control – the room becomes isolated from the central system
- Only air having passed through both UVC and HEPA filtration will be returned to hospital HVAC
IsolationAir® meets CDC, AIA and ASHRAE guidelines for new or renovation
- 12 air changes per hour via HEPA filters
- Each IsolationAir unit conditions rooms up to 375 sqft with an 8’ ceiling
- Pressure differential of 0.01” minimum between room and adjoining spaces
(May require additional seals around doors or other significant leak points in large rooms with poorly sealed doors) - Continuous operation when plugged into emergency generator outlet
- Provides stable temperature control for patient comfort
- Originally designed to meet the U.S. Department of Health and Human Services’ critical benchmarks:
- Critical Benchmark #2-2: Surge Capacity: Isolation Capacity
- Critical Benchmark #2-9: Surge Capacity: Trauma and Burn Care
- Cross-cutting Critical Benchmark #6: Preparedness for Pandemic Influenza
View Testing for Usage in Surge Capacity Hospital Rooms Study
View Portable Surge Capacity Testing at BIC Report
View Portable Surge Capacity Testing at BIC Poster
View Air Innovations and the CARES Act Reimbursement Program Page
Airborne Infection Isolation Rooms (AIIR) (Negative Pressure Setup)
Protective Environments (Positive Pressure Set Up)