From Our Customers:
“Thank you so much. I really like the cabinets and they have helped us get into compliance.” Government Customer, “K.E.”
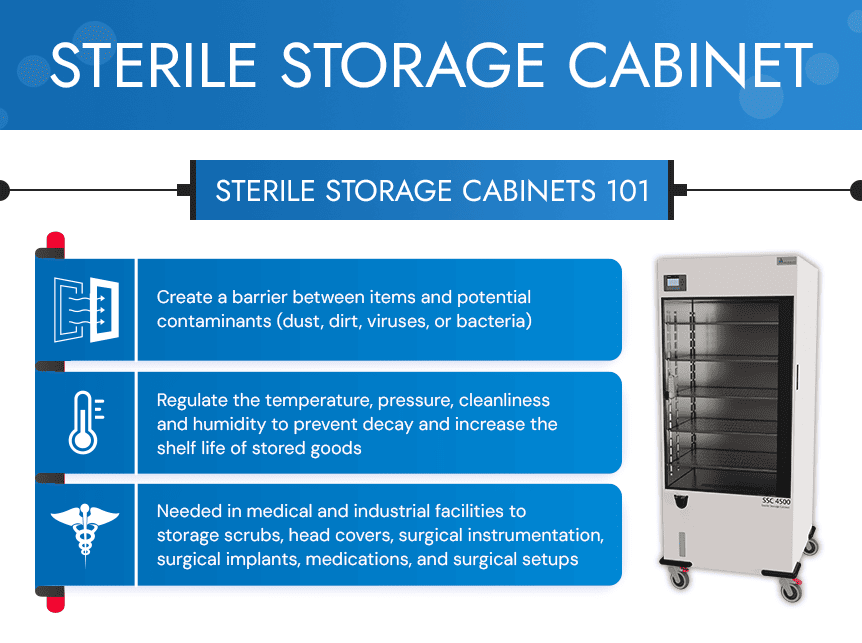
Ensuring Sterile Storage Conditions
Sterile storage cabinets offer optimal conditions for storing goods to maintain their existing level of cleanliness after they undergo sterilization or while they are in their original sealed packaging. Look for cabinets that offer the following:
- Flexibility
- Temperature and Humidity control
- Positive Pressure
- Filtration
- Cleanliness
Well-designed cabinets drastically reduce the risk of contamination even after the equipment has been sterilized. Some of these events include excessive handling, moisture penetrating the item’s package or seal, or exposure to contaminants.
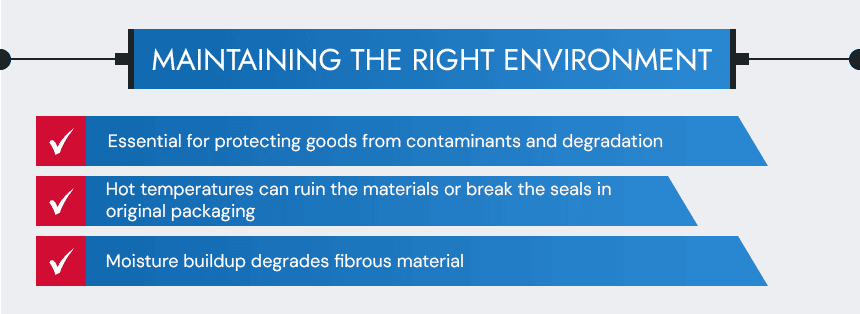
Sterile Storage Temperature and Humidity Parameters
Maintaining the right temperature and humidity conditions is essential for protecting goods from contaminants and degradation. Hot temperatures can ruin or warp materials, break the seals in packaging, or denature biological samples and specimens. Moisture buildup degrades fibrous material, causes rust, and can create a breeding ground for even small traces of microorganisms. While this is a risk for even short-term storage, it is an even more pressing concern for long-term storage with irregular access.
Facilities can manage these risks by investing in ventilated storage cabinets that keep the temperature below 75°F and humidity levels between 20% and 60%. These cabinets should be augmented with sensors and chemical indicators that can alert facility managers of potential exposure or changes to the storage conditions.
Learn More About Our Sterile Storage Solution.
Sterile Storage Cabinet Features:
At Air Innovations, we build our cabinets to replicate cleanroom conditions, so you can easily monitor the condition of the contents and rely on the integrity of the space. Each of our portable cabinets offers steam humidification to meet ideal storage conditions with just standard demineralized water, and each system can interface with nearly any network environmental monitoring systems, including FreshLoc or TempTrak systems. The cabinets have features such as metal construction, industry-standard filtration and humidity control, smart monitoring tools that limit traffic or exposure, and more:
- Bottom casters for easy movement
- Hospital-grade 304 stainless steel construction that’s easy to sanitize
- HEPA Filtration designed to exceed ISO 4 Cleanliness (or better in controlled environments)
- 120V hospital-grade 10ft long plug-in cord
Our cabinets meet or exceed the following standards:
- IEC 60601-1 Medical Standard for Power Supplies
- ISO 4 Cleanliness (or better in controlled environments)
- Requirements of VA Directive 1116(2);
—CSA (Canadian Standards Association) Z314.15-10;
—ANSI/AAMI ST79-2006 (by storing sterilized instruments in an environment with temperature and humidity control, positive pressure, HEPA filtration, and ventilation);
—ANSI/ASHRAE/ASHE Standard 170-2017
Contact Us for Your Sterile Storage Needs
Having access to the right storage keeps your equipment, patients, and business safer. We have leasing options available, so you can store your items when you need it the most. Contact us today to find the right sterile storage cabinet that fits your requirements.
Leasing Options Available
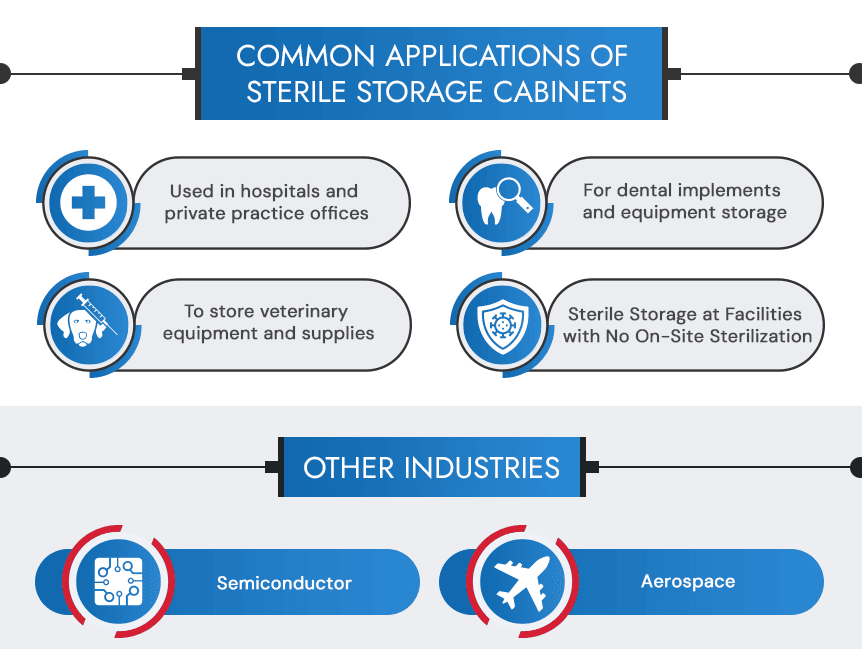
Common Applications
SSC4500 Sterile storage cabinets belong wherever facilities need access to sterilized personal protective equipment (PPE), reliable inventory storage of humidity and temperature-sensitive goods, and medical implements. Some of the most popular applications of our sterile storage cabinets include the following:
- Use in hospitals and private practice offices
- For critical component and equipment storage
- To store veterinary equipment and supplies
- Retrofitting facilities with additional cleanroom storage
- Offsite storage of single-use, manufacturer sterilized supplies
Interested in learning about how our Sterile Storage Cabinet came to be? Read our case study regarding the process of designing and manufacturing the product.
Note: The SSC4500 is not intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention, of disease in humans or in other animals.
Contact Us today to learn more and to discuss your post sterile storage requirements!